What are the physical mechanisms that lead to broadening? We’ll discuss three here.
The first is called lifetime broadening. As you know, quantum mechanics tells us that the electron wavefunction is oscillating at frequency $latex \nu = E / h$, where energy $latex E$ is determined by the state that the electron occupies. As we discussed in the context of light, a wave can only be truly single-frequency if it oscillates for all time. An electron in an energy state has a finite lifetime in that state; eventually it will move to another state, by spontaneous processes or otherwise. Therefore, each state has a finite lifetime, sometimes labelled $latex \tau$. The finite duration of the wavefunction’s oscillation necessarily introduces additional frequency (energy) components to the electron’s wavefunction, thus broadening its spectrum. This form of broadening can’t be avoided, but it also produces very narrow broadening in practice, as many states have very long lifetimes compared to their oscillation frequency.
The second broadening mechanism we’ll discuss is called collision broadening. When we discussed incoherent light, we described random phase jumps that cause a wave to lose phase information. These random phase jumps can also occur for electron wavefunctions, commonly due to elastic collisions with other atoms (or lattice vibrations, in the case of solid state materials). The random phase jumps will introduce additional frequency components and therefore broaden the spectrum of the electron’s wavefunction. This broadening can be significant especially at high temperatures in certain materials.
The last broadening mechanism we’ll discuss is Doppler broadening. You’re probably familiar with the Doppler effect – as a train approaches, the whistle sounds higher-pitched than when it recedes. There is also a Doppler effect for light, although the math is a bit different owing to the fact that light must always pass you at the same speed. If you detect light from a source that is moving towards you, you will measure a shorter wavelength than you would detect from an identical source that is moving away from you. In a gas laser, the light-emitting atoms are moving in random directions; at any moment, some are approaching you while others move away from you, in roughly equal numbers. Therefore you will observe broadening in the spectrum of light emitted by this collection of atoms, due to the differing Doppler shifts that the light emitted by different atoms experience.
While we’re discussing broadening mechanisms, we can divide all broadening mechanisms into two broad categories: homogeneous broadening and inhomogeneous broadening. Let’s consider a collection of light emitters, like atoms in a gas. The gas is homogeneously broadened if all of the atoms in the gas are broadened in the same way – i.e. the emission spectrum of each atom is the same, with the same center frequency and linewidth. In this case, the overall broadening of the gas is the same as the broadening of each atom. For a gas composed of a single species of atoms, which are all subjected to the same environmental conditions, we would expect that lifetime and collision broadening mechanisms would result in homogeneous broadening.
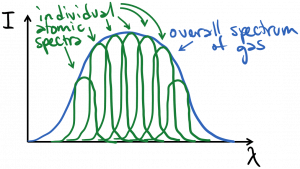
In a gas that is inhomogeneously broadened, different light emitters in the gas may have different spectra – a different center frequency and/or linewidth. The overall spectrum of the collection of emitters is therefore a sum or composite of the varied spectra of the individual emitters, as illustrated below. A gas with Doppler broadening as the dominant mechanism would be inhomogeneously broadened, as each atom would effectively have a different center frequency, dependent upon their velocity.


You must be logged in to post a comment.