I mentioned that photons can be absorbed by electrons, which are then boosted in energy by an amount equal to the photon energy:
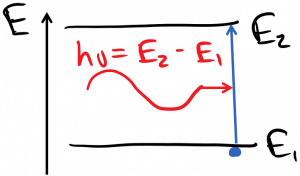
and photons can be spontaneously emitted by electrons, which then lose an amount of energy equal to the photon energy:
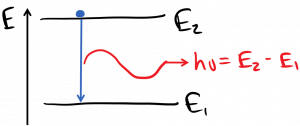
This emission process is called ‘spontaneous emission’, because it happens spontaneously: an electron in a higher energy state spontaneously emits a photon and drops to a previously empty lower energy state. Thermodynamics tells us that in equilibrium, we should not expect to find many atoms whose electrons are in upper energy states while lower energy states are empty, but as long as the temperature is greater than absolute zero, there will be a few.
There is another emission process, originally posited by Einstein and validated experimentally since, which is the most important process for a laser: stimulated emission. In this case, the atom acts a bit like a photocopier or a cloning machine. An incoming photon stimulates an already-excited atom to emit another photon, which will have the same frequency, phase, polarization, and direction as the stimulating photon:
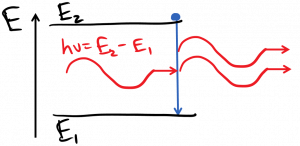
This one is generally a bit confusing at first, but we should think of the atom like a driven oscillator. The incoming light wave acts like a driving field, and pushes the excited atom to oscillate in step with this field, so it’s no surprise that the atom then emits a photon in step with the driving field.


You must be logged in to post a comment.